Abstract
Background. Hereditary chronic hemolytic anemias (CHAs) is an heterogeneous group of rare disorders including defects of the RBC membrane (hereditary spherocytosis (HS), elliptocytosis (HE), pyropoikilocytosis (HPP), or defects of cation permeability, hereditary stomatocytosis (HSt)), defects of RBC metabolism (affecting glycolysis, the pentose-phosphate shunt or nucleotide metabolism), hemoglobinopathies, and disorders of erythropoiesis (e.g. congenital dyserythropoietic anemia, CDAs). All these diseases are complex and frequently difficult to diagnose. The laboratory tools for the diagnosis of CHAs include routine tests but also require more specialized analyses, available only in few dedicated laboratories. In our experience, a conclusive diagnosis is not reached in about 15-20% of cases despite detailed and exhaustive investigations. Genetic analysis is not widely used in these disorders due to the clinical and molecular heterogeneity, and is usually performed only when the hematologic diagnostic workup gives a clear diagnostic orientation. The advent of next generation sequencing (NGS) methods however has made diagnosing complex genetic disorders feasible.
Aim. We developed and applied a NGS targeted panel to identify the underlying genetic cause in a selected series of patients with undiagnosed CHAs.
Methods. A total of 35 cases with CHAs from 26 families were studied. In 21 subjects no definitive diagnosis was previously done despite extensive investigation, in 7 the conventional diagnostic workup was unreliable due to transfusions or sample shipping. In addition three HS/HE families (7 cases) with intra-family clinical variability were studied to elucidate the molecular basis of the atypical phenotype. Using SureDesign software (Agilent) we created a NGS based panel containing 40 genes associated with RBC membrane disorders (13 genes), enzymopathies (20), CDAs (7), and 9 other candidate or modifier genes. Libraries were obtained by HaloPlexHS Target Enrichment System Kit and sequenced on a MiSeq platform (Illumina). The panel was validated on previously characterized CHAs patients. Mutations were confirmed by Sanger method.
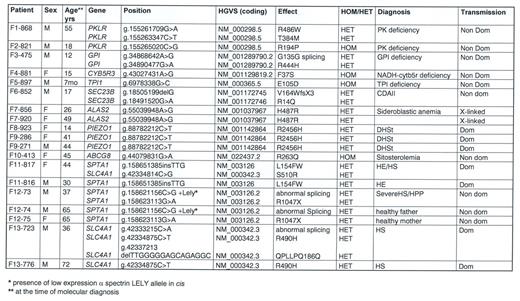
Results. We identified pathogenic variants that enabled a definitive diagnosis in 19 cases (13 families). Results are reported in Table 1.
RBC enzyme defects were identified in 5 patients: two cases of pyruvate kinase deficiency (one of them with normal PK activity in absence of transfusions), one case of glucosephosphate isomerase deficiency, and one of NADH-CytB5R deficiency (in a case with methemoglobinemia). Notably, we were able to detect a triosephosphate isomerase deficiency in a 7 month child with anemia but no neuromuscular manifestations at the time of the study, thus permitting an early diagnosis in this rare disease.
Dyserythropoietic anemias. SEC23B gene mutations associated with CDAII were identified in one patient. A new pathogenic variant in ALAS2 gene was detected in a female with macrocytic anemia, suggesting a diagnosis of congenital sideroblastic anemia; HUMARA analysis showed a skewed X-Chr inactivation pattern; the mutation was transmitted by the asymptomatic mother.
RBC membrane defects. Three cases carried the known pathogenic variant p.R2456H in PIEZO1 gene, associated with hereditary xerocytosis. A mutation in ABCG8 gene associated with congenital sitosterolemia was detected in case F10-413, who had a long-lasting diagnosis of overhydrated HSt suggested by a right shifted ekacytometric curve.
Complex genotypes were identified in 3 families with HS/HE: case F11-817 (mixed HS/HE phenotype) carried two pathogenic variants in SPTA1 and SLC4A1, whereas the brother with typical HE had only the SPTA1 variant; case F12-73 with HPP and combined 68% spectrin and 56% ankyrin deficiency, had two different in trans mutations in SPTA1, one of them associated in cis with the alpha-LELY allele. Finally, case F13-723 (severe HS with 50% band 3 deficiency) had 3 different variants in SLC4A1 gene, the father presenting only one missense mutation, thus justifying intra-family clinical variability.
Conclusions. NGS platform is a powerful tool to elucidate the molecular causes in patients with CHAs where traditional hematologic testing failed, leading in this series to the clarification of 50% of the examined families. Extensive clinical and hematologic information is needed to support the interpretation of molecular data.
van Wijk: Agios Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Barcellini: Alexion: Honoraria; Agios: Honoraria, Research Funding; Novartis: Honoraria. Bianchi: Agios Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.